How to use the BP
The British Pharmacopoeia (BP) is the only comprehensive collection of authoritative official standards for UK pharmaceutical substances and medicinal products. It contains all texts and monographs of the European Pharmacopoeia (signposted with a chaplet of stars), as well as the national standards developed by the BP. You can find more information about European Pharmacopoeia (Ph. Eur.) texts in Supplementary Chapter IV. European Pharmacopoeia.
This guide is designed to help you become more familiar with the requirements of the BP, where to locate the different types of information and how to use and interpret the requirements of a formulated preparation monograph. We value your feedback as users of the BP, if you would like to suggest improvements and changes to the guide, please email us at bpcom@mhra.gov.uk.
Getting started
If a drug product is licensed in a country where the BP is a legal standard, it should:
- be able to comply with the requirements of the BP at any time throughout its shelf life
- meet the standards of the BP regardless of whether compliance with the BP is claimed or it is not called by the name at the head of the monograph.
For a drug product to be compliant with a BP monograph:
- the monograph that was in force at the date of product manufacture should be applied e.g. BP 2026 is legally effective between 01/01/2026 and 31/12/2026. The effective date an be found in the Introduction section of the BP
- all ingredients (drug substances and excipients) that are used to make the product should comply with the published BP or Ph. Eur. monograph for those substances
- the product should comply with the relevant general monographs including the general monograph appropriate for the dosage form in question e.g. Pharmaceutical Preparations, Tablets and Tablets of the British Pharmacopoeia.
If you:
- have developed a method which is an improvement on the current BP procedure and want to propose a revision to the monograph
- believe that there is an error in the BP
contact bpcom@mhra.gov.uk with the information needed to contribute to monograph development (see Contribute to monograph development and improvement), providing details of the error with data if appropriate.
Navigating the BP
Guidance on where certain types of information is located within the BP is given below:
BP CONTENT
Preliminaries
The Preliminaries (Volume I of the print version) consist of several informative texts, including the Introduction which details the new, revised and omitted monographs in any given edition of the BP, the legally effective date and an update on high profile BP activities.
General Notices
The General Notices contain information applicable to all texts in the BP and are essential reading. General notices explain which statements are mandatory and non-mandatory, and the definition of terms. They can be found in the online BP using the 'Table of Contents' or following the 'General Notices' link in monographs. The General Notices are the pink pages at the front of each volume of the print version.
Monographs: Medicinal and Pharmaceutical Substances
Monographs: Medicinal and Pharmaceutical substances (Volumes I and II of the print version) contains monographs for active pharmaceutical ingredients and excipients.
Formulated Preparations: General Monographs
All pharmaceutical products should comply with the general monographs for pharmaceutical preparations and the dosage form (the product should also comply with the specific preparation monograph where one is published). These are found in Formulated Preparations: General Monographs online (Volume III of the print version). For some dosage forms (Topical Powders, Capsules, Tablets, Eye Preparations, Nasal Preparations, Liquids for Cutaneous Application, Topical Semi-solid Preparations, Oral Liquids and Parenteral Preparations) there will be additional BP requirements provided in a separate general monograph.
Formulated Preparations: Specific Monographs
Monographs for specific drug formulations, incorporating small molecule, biological and unlicensed medicines, can be found in this section of the online BP (Volume III of the print version). A more detailed explanation of a formulated preparation BP monograph can be found in the next section of this guide.
Appendices
The Appendices (Volume V of the print version) contain methods of analysis and calibration requirements for analytical techniques (Ph. Eur. section 2), texts relating to containers (Ph. Eur. section 3), reagents and volumetric solution preparation (Ph. Eur. section 4) and the majority of the general texts of Ph. Eur. section 5. When an appendix is referenced in a monograph, the requirements of that appendix are mandatory unless stated otherwise. The European Pharmacopoeia Equivalent Texts page correlates Ph. Eur. texts to the Appendix reference of the BP.
Herbal Drugs, Herbal Drug Preparations and Herbal Medicinal Products / Materials for use in the Manufacture of Homoeopathic Preparations / Blood-related Products / Immunological Products / Radiopharmaceutical Preparations / Surgical Materials
All monographs relating to herbal drugs and homeopathics, blood related products, immunological products and vaccines, radiopharmaceuticals and surgical materials, can be found from the relevant section in the online table of contents (Volume IV of the print version).
Infrared Reference Spectra
There is a link to relevant infrared reference spectra in formulated preparation monographs (Volume V of the print version). Determining concordance of IR spectra is explained in the General Notices.
Supplementary Chapters
The Supplementary Chapters contain non-mandatory information and guidance. If you need advice to understand a test or calculate results in the BP, the supplementary chapters may contain the information you are looking for (Volume V of the print version).
British Pharmacopoeia (Veterinary)
The BP (Vet) contains the sections described above for pharmaceutical and medicinal products that are only used in veterinary medicine (Volume VI of the print version).
Advanced Therapy Medicinal Products (ATMPs) Guidance
The Advanced Therapy Medicinal Products (ATMPs) guidance contains non-mandatory guidance on best practices for helping ensure quality of cell and gene therapies. The guidance is available to all users of the digital products and is also freely accessible on the BP website. For more information and to access the guidance, please visit the Advanced Therapy Medicinal Products Guidance page.
Following a monograph
This section is designed to provide a broad explanation of how to apply and interpret a BP formulated preparation monograph, using the BP 2026 Atorvastatin Tablets monograph as an example.
When using a monograph for a drug product:
- you should read the General Notices; these form the foundation of pharmacopoeial requirements and define the terms used in monographs
- you should apply the requirements of the general monograph for Pharmaceutical Preparations and the general monographs for the particular dosage form, e.g. for tablets, these are Tablets (Ph. Eur. monograph 0478) and Tablets of the British Pharmacopoeia. These can be found in the Formulated Preparations: General Monographs section in the publication table of contents
- the analytical techniques used should meet the specifications and requirements set out in the relevant appendices e.g. Appendix III Chromatographic Separation Techniques and Appendix III D Liquid Chromatography when using LC. Appendices form part of the official requirements of the BP where specified in a monograph. Supplementary Chapters are explanatory texts provided for information and to assist users
- you should confirm that the methods are suitable for the particular drug product being tested e.g. that excipients in the product do not affect the analysis
- you are not precluded from using alternative methods, provided that you are able to demonstrate that it gives an equivalent measure of the requirement. This is stated in the General Notices Part II, in the section on 'Assays and Tests'.
Monograph example with explanatory notes
Atorvastatin Tablets
(Explanation 1 - opens in a modal)General Notices (Explanation 2 - opens in a modal)
Action and use
(Explanation 3 - opens in a modal)HMG Co-A reductase inhibitor; lipid-regulating drug.
DEFINITION
(Explanation 4 - opens in a modal)Atorvastatin Tablets contain Atorvastatin Calcium.
The tablets comply with the requirements stated under Tablets(Explanation 5 - opens in a modal) and with the following requirements.
Content of atorvastatin, C33H35FN2O5
95.0 to 105.0% of the stated amount. (Explanation 6 - opens in a modal)IDENTIFICATION
(Explanation 7 - opens in a modal)In the Assay, record the UV spectrum of the principal peak in the chromatograms obtained with solutions (1) and (2) with a diode array detector in the range of 210 to 400 nm.
The UV spectrum of the principal peak in the chromatogram obtained with solution (1) is concordant with that of the peak in the chromatogram obtained with solution (2);
the retention time of the principal peak in the chromatogram obtained with solution (1) is similar to that of the peak in the chromatogram obtained with solution (2).
TESTS
(Explanation 8 - opens in a modal)Dissolution
Comply with the dissolution test for tablets and capsules, Appendix XII B1
Test conditions
Procedure
Determination of content
Calculate the total content of atorvastatin, C33H35FN2O5, in the medium from the absorbances obtained and using the declared content of 2(C33H34FN2O5),Ca in atorvastatin calcium BPCRS Each mg of 2(C33H34FN2O5),Ca is equivalent to 0.9671 mg of C33H35FN2O5.
Limits
(Explanation 10 (opens in a modal))The amount of atorvastatin released is not less than 75% (Q) of the stated amount.
Related substances
(Explanation 11 - opens in a modal)Carry out the method for liquid chromatography, Appendix III D, using the following solutions prepared in solution A.
Solution A Equal volumes of acetonitrile and 0.05m citric acid, previously adjusted to pH 7.4 with dilute ammonia R1
Chromatographic conditions
(Explanation 14 - opens in a modal)Mobile phase
Solution B 75 volumes of stabiliser-free tetrahydrofuran and 925 volumes of acetonitrile.
Solution C 0.05m ammonium dihydrogen orthophosphate, previously adjusted to pH 4.3 with dilute acetic acid or dilute ammonia R1.
Mobile phase A 42 volumes of solution B and 58 volumes of solution C.
Mobile phase B 20 volumes of solution B, 20 volumes of solution C and 60 volumes of methanol.
|
Time (Minutes)
|
Mobile phase A (% v/v)
|
Mobile phase B (% v/v)
|
Flow rate (mL per minute)
|
Comment
|
|---|---|---|---|---|
|
0-30
|
100
|
0
|
1.8
|
isocratic
|
|
30-45
|
100→25
|
0→75
|
1.8
|
linear gradient
|
|
45-50
|
25
|
75
|
1.5
|
isocratic
|
|
50-55
|
25→20
|
75→80
|
1.5
|
linear gradient
|
|
55-58
|
20→100
|
80→0
|
1.8
|
linear gradient
|
|
58-65
|
100
|
0
|
1.8
|
re-equilibration
|
System suitability
(Explanation 16 - opens in a modal)The test is not valid unless:
in the chromatogram obtained with solution (3), the resolution between the peaks due to atorvastatin and impurity B is at least 1.4;
in the chromatogram obtained with solution (5), the signal-to-noise ratio of the peak due to atorvastatin is at least 20(Explanation 17 - opens in a modal).
Calculation of impurities
(Explanation 18 - opens in a modal)For each impurity, use the concentration of atorvastatin in solution (2)(Explanation 19 - opens in a modal).
For the reporting threshold, use the concentration of atorvastatin in solution (5).
For peak identification, use solutions (3) and (4).
Atorvastatin retention time: about 19 minutes(Explanation 20 - opens in a modal).
Relative retention: impurity A, about 0.85; impurity 1, about 0.9; impurity B, about 0.95; impurity H, about 2.0; impurity 2, about 2.1; impurity 3, about 2.3; impurity 4, about 2.4.
Correction factors: (Explanation 21 - opens in a modal) impurity 1, multiply by 1.5; impurities 2 and 3, multiply by 1.9.
Limits
(Explanation 22 - opens in a modal)ASSAY
Weigh and powder 20 tablets. (Explanation 23 - opens in a modal) Carry out the method for liquid chromatography, Appendix III D, using the following solutions prepared in solution A.
Solution A Equal volumes of acetonitrile and 0.05m citric acid, previously adjusted to pH 7.4 with dilute ammonia R1.
Chromatographic conditions
Mobile phase
20 volumes of stabiliser-free tetrahydrofuran, 27 volumes of acetonitrile and 53 volumes 0.05m citric acid, previously adjusted to pH 4.0 using dilute ammonia R1.
When the chromatograms are recorded under the prescribed conditions, the relative retention with reference to atorvastatin (retention time about 9 minutes) is impurity H, about 1.3.
System suitability
(Explanation 25 - opens in a modal)The test is not valid unless, in the chromatogram obtained with solution (3), the resolution between the peaks due to atorvastatin and impurity H is at least 5.0.
Determination of content
Calculate the content of atorvastatin, C33H35FN2O5, in the tablets from the chromatograms obtained and using the declared content(Explanation 26 - opens in a modal) of 2(C33H34FN2O5),Ca in atorvastatin calcium BPCRS.
Each mg of 2(C33H34FN2O5),Ca is equivalent to 0.9671 mg of C33H35FN2O5.
Analytical Quality by Design concepts were applied in the development of this Assay, Supplementary Chapter X on the use of Analytical Quality by Design concepts for analytical procedures. The "More resources" tab, available in the BP online monograph, provides additional information on the ranges for sample preparation and chromatographic parameters that were found to be suitable, as well as method performance, to aid user investigations into method suitability.
LABELLING
(Explanation 28 - opens in a modal)The quantity of active ingredient is stated in terms of the equivalent amount of atorvastatin.
IMPURITIES
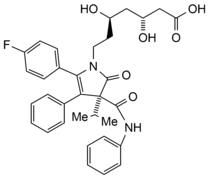
![2. 4-[7-(4-fluorophenyl)-7-hydroxy-7a-phenyl-1a-(phenylcarbamoyl)-1b-(propan-2-yl]hexahydro-3H-oxireno[3,4]pyrrolo[2,1-b][1,3]oxazin-3-yl)-3-hydroxybutanoic acid (epoxy pyrrolooxazin 6-hydroxy analogue)](/content/images/atorvastatintablets_2_bp2023_cs_small.png)
![3. 4-[1b-(4-fluorophenyl)-7-hydroxy-1a-phenyl-7a-(phenylcarbamoyl)-7-(propan-2-yl)hexahydro-3H-oxireno[3,4]pyrrolo[2,1-b][1,3]oxazin-3-yl]-3-hydroxybutanoic acid (epoxy pyrrolooxazin 7-hydroxy analogue)](/content/images/atorvastatintablets_3_bp2023_cs_small.png)
![4. 4-(4-fluorophenyl)-2,4-dihydroxy-2-isopropyl-N,5-diphenyl-3,6-dioxabicyclo[3.1.0]hexane-1-carboxamide (epoxy THF analogue)](/content/images/atorvastatintablets_4_bp2023_cs_small.png)
Explanations
Show all explanationsTimeline
The timeline and revision history tab (available for digital users only) details when a monograph was first published and shows a timeline of any previous versions of a monograph. You can find out more information on how to use this feature in our ‘How to get the most out of your subscription’ guide, accessible on the BP online features page.
Monographs in this edition of the BP will become legally effective from 1st January 2026. You should use the version of the monograph that is effective at the date of product manufacture.
1. Official title
The official title of the product in the UK. Atorvastatin is the recommended international non-proprietary name (rINN), designated by the World Health Organization (WHO), and subsequently adopted as the British Approved Name (BAN).
2. The General Notices
Links to General Notices
- provide useful information on using the BP
- explain that a product should comply with the monograph, if tested
- explain which sections of the monographs are mandatory & which are advisory
- explain that alternative tests to the ones described in the monograph can be used.
3. Action and use
Advisory information on the most common indication(s). This is not exhaustive and is usually aligned with the entry in the British Approved Names (BAN) publication.
Return to "Action and use" example4. Definition
The drug substance used to manufacture the tablets must comply with the relevant Ph. Eur. or BP monograph for that substance. In this case the Atorvastatin Calcium monograph (Ph. Eur. monograph 2191, Atorvastatin Calcium Trihydrate).
Return to "Definition" example5. Tablets general monograph
Links to Tablets
The tablets must comply with the Tablets general monograph.
Return to "Tablets general monograph" example6. Tablets contents
The tablets must contain 95.0 to 105.0% of the amount stated on the label for the shelf life of the product. The content is written in terms of the label claim of the product, which may not match the full title of the drug substance monograph. This is common when the drug substance used to manufacture the product is in a salt form.
Return to "Tablets contents" example7. Identification
BP monographs are designed for products that are manufactured within the quality framework for medicinal products. When applied within this framework, the identification test (or tests) in the monograph are sufficient to confirm that the drug product contains the drug substance on the label.
Return to "Identification" example8. Tests
The tests contained in this section of the monograph are to determine quality attributes of the product. The product must comply with the requirements of the tests.
The methods in the monograph are the official methods which support the standard. However, alternative methods can be used if the user can demonstrate that it gives an equivalent measure of the requirement. This is stated in the General Notices Part II, in the section on ‘Assays and Tests’.
Return to "Tests" example9. Links to other BP sections
Links to sodium hydroxide
Where a word/phrase is in italics, it means it is connected to another section of the BP. In this case, the reagent entry for sodium hydroxide in Appendix I A. General Reagents. The reagent used should comply with the criteria stated in the appendix entry.
Return to "Links to other BP sections" example10. Limits
Additional advice on dissolution testing can be found in SC I E. Dissolution Testing of Solid Oral Dosage Forms, including worked examples for applying harmonised dissolution limits (Q values).
Return to "Limits" example11. Related substances
This test is designed to provide limits for potential impurities related to the drug substance, rather than all possible impurities such as adulterants or contaminants.
Return to "Related substances" example12. EPCRS and CRS
Links to atorvastatin for peak identification A EPCRS
Reference material with the suffix EPCRS and CRS can be obtained from the European Directorate for the Quality of Medicines & HealthCare (EDQM).
Return to "EPCRS and CRS" example13. BPCRS Information Leaflet
Links to atorvastatin impurity standard BPCRS
BPCRS Information Leaflets can be a good source of guidance e.g. reference chromatograms. Information leaflets are available free of charge in the online reference standards catalogue. For online BP users, the BPCRS details tab above the monograph title will take you directly to the relevant BPCRS catalogue items used in the monograph.
Return to "BPCRS Information Leaflet" example14. Chromatographic conditions
Appendix III Chromatographic Separation Techniques includes details on making adjustments to chromatographic conditions.
Return to "Chromatographic conditions" example15. Column brand
The brand of the column that was used when the monograph method was established is provided for information to aid the analyst.
Return to "Column brand" example16. System suitability
Appendix III Chromatographic Separation Techniques gives details of how to measure resolution, signal-to-noise and peak-to-valley ratios. This Appendix also includes standard system suitability requirements for analytical procedures, such as system sensitivity and peak symmetry. These standard requirements are not reproduced in each individual monograph unless a modification or exemption to the requirement is permitted.
Return to "System suitability" example17. Signal-to-noise ratio
The target value of the signal-to-noise ratio for the reporting threshold solution might be affected by impurities’ correction factors. Here, the signal-to-noise ratio of 20 is an exemption to the normal requirement of 10 due to the correction factors applied to impurities 1-3.
Return to "Signal-to-noise ratio" example18. Calculation Of impurities
This section is designed to provide relevant information for the quantitative limits calculation. Quantitative limits started to be included in BP monographs from BP 2023 onwards. This approach makes it easier to adopt BP methods for routine impurity testing and monitoring. The terminology used in these tests is aligned with ICH.
Return to "Calculation Of impurities" example19. Calculation against reference solution
This instruction informs a user that each impurity should be calculated against the known concentration of Atorvastatin in a reference solution. The reference solution may be a dilution of the test solution or a reference substance. The external standard method of quantitation is used, which is outlined in Appendix III Chromatographic Separation Techniques. Use the stated solution if adopting the one-point calibration approach.
Return to "Calculation against reference solution" example20. Retention time variation
It is important to note the word ‘about’. If the system suitability requirement (below) is met, and the impurities can be detected/identified, the retention time does not need to be exactly 19 minutes. There is likely to be variation due to the use of different equipment and columns. Check the More Resources section in the online BP for example test results (if there are some available).
Return to "Retention time variation" example21. Correction factors
Correction factors are used to adjust for differences in response between the impurity of interest and the substance it is being measured against. Correction factors are included if they are outside of the range 0.8 - 1.25.
Return to "Correction factors" example22. Limits
Guidance on the control of impurities and on calculating limits can be found in SC I A. Control of Impurities and SC VI A. Pharmacopoeial Calculations. A worked example of the calculation of quantitative impurity results can be found in SC VI A.
Return to "Limits" example23. Assay
20 units is traditionally accepted as a statistical representation of a batch. This is explained in the general monograph Tablets of the British Pharmacopoeia.
Return to "Assay" example24. Ambient temperature
The General Notices require that, unless stated otherwise, assays and tests are performed between 15 to 25 °C. Ambient temperature should be taken to mean between 15 to 25 °C.
Return to "Ambient temperature" example25. System suitability
Where an additional point for monographs of the British Pharmacopoeia is in place, these can be found at the end of the relevant text. For assays of formulated preparations, a system suitability point can be found in Appendix III, which states that the the maximum permitted relative standard deviation for six replicate injections of the prescribed reference solution does not exceed 2.0%.
Return to "System suitability" example26. Declared content
The declared content of a BPCRS can be found in the online catalogue and in the leaflet.
Return to "Declared content" example27. Supporting information for assay
The Assay for the Atorvastatin Tablets monograph was the focus of a case study to demonstrate the practical application of Analytical Quality by Design (AQbD) principles to the development of an analytical procedure.
The additional method understanding gained through the application of these approaches is detailed under the “More resources” tab. Under this tab you may also find example chromatograms and examples of how to reduce the environmental impact of the methods.
You can read more about our AQbD and Sustainability guidance via the About BP guidance materials page.
Return to "Supporting information for assay" example28. Labelling
Labelling requirements in monographs are not comprehensive and the requirements of the regulatory authority where the product is marketed should be met. More information on the status and interpretation of the labelling section can be found in SC I G. Labelling.
Return to "Labelling" example29. Impurities
This means that the Related substances test can detect and control the named impurities listed in the drug substance monograph for Atorvastatin Calcium (Ph. Eur. monograph 2191, Atorvastatin Calcium Trihydrate). Where there are impurities that can be controlled and that are not stated in the drug substance monograph, these impurities are given a number and listed in the monograph.
Return to "Impurities" example